Are you ready to discover 'write a balanced equation for the combustion of 1 of c8h18'? You will find your answers right here.
Table of contents
- Write a balanced equation for the combustion of 1 of c8h18 in 2021
- Combustion of gasoline equation
- Write a balanced chemical equation for the combustion of octane (c8h18)
- C8h18 + o2 incomplete combustion
- What type of reaction is c8h18+o2=co2+h2o
- C8h18+o2=co2+h2o balanced equation
- Incomplete combustion of octane equation
- C8h18 combustion reaction
Write a balanced equation for the combustion of 1 of c8h18 in 2021
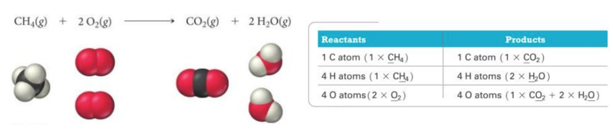 This image illustrates write a balanced equation for the combustion of 1 of c8h18.
This image illustrates write a balanced equation for the combustion of 1 of c8h18.
Combustion of gasoline equation
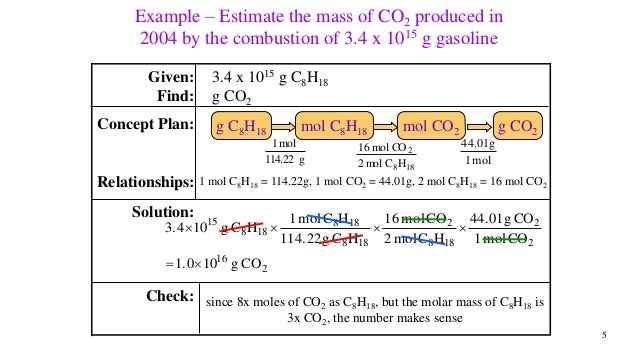 This picture shows Combustion of gasoline equation.
This picture shows Combustion of gasoline equation.
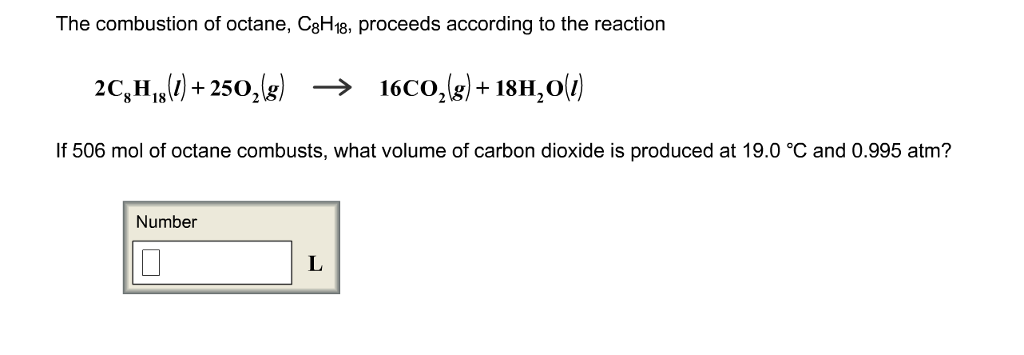
Write a balanced chemical equation for the combustion of octane (c8h18)
 This picture shows Write a balanced chemical equation for the combustion of octane (c8h18).
This picture shows Write a balanced chemical equation for the combustion of octane (c8h18).
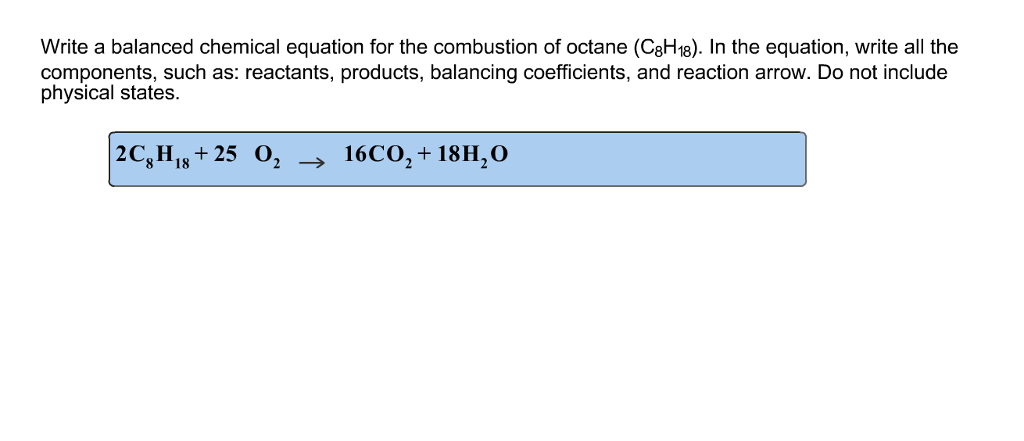
C8h18 + o2 incomplete combustion
 This picture shows C8h18 + o2 incomplete combustion.
This picture shows C8h18 + o2 incomplete combustion.
What type of reaction is c8h18+o2=co2+h2o
 This picture representes What type of reaction is c8h18+o2=co2+h2o.
This picture representes What type of reaction is c8h18+o2=co2+h2o.
C8h18+o2=co2+h2o balanced equation
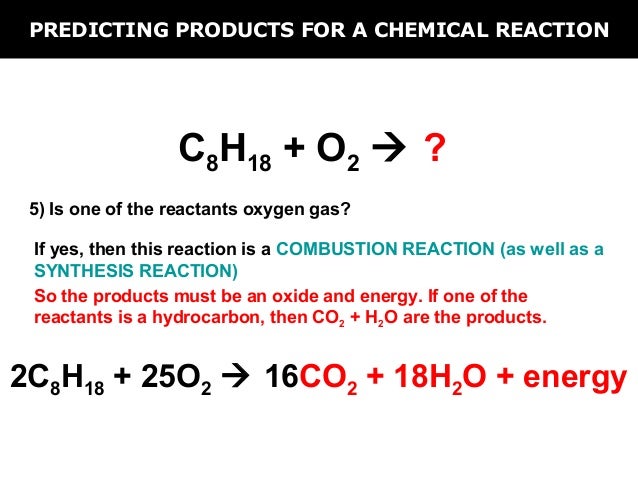 This image demonstrates C8h18+o2=co2+h2o balanced equation.
This image demonstrates C8h18+o2=co2+h2o balanced equation.
Incomplete combustion of octane equation
 This picture shows Incomplete combustion of octane equation.
This picture shows Incomplete combustion of octane equation.
C8h18 combustion reaction
 This image representes C8h18 combustion reaction.
This image representes C8h18 combustion reaction.
How to balance C8H18 + O2 with CO2?
In order to balance C8H18 + O2 = CO2 + H2O you'll need to watch out for two things. First, be sure to count all of C, H, and O atoms on each side of the chemical equation. Click to see full answer.
What is the correct chemical equation for the complete combustion?
The general equation for a complete combustion reaction is: Fuel + O2 → CO2 + H2O. The burning of charcoal is a combustion reaction. Similarly one may ask, what is the correct balanced chemical equation for the complete combustion of octane c8h18? Thus we can balance the oxygen atoms by putting a prefix of 25/2 on the left side.
What is the complete balanced reaction for the complete...?
What is the complete balanced reaction for the complete combustion of c8h18 in oxygen? In order to balance C8H18 + O2 = CO2 + H2O you'll need to watch out for two things. First, be sure to count all of C, H, and O atoms on each side of the chemical equation. Click to see full answer.
How to write a balanced equation for octane combustion?
How would you write a balanced equation for the combustion of octane, C8H18 with oxygen to obtain carbon dioxide and water? You just have to check the number of atoms of each element is the same in both sides. By making a list of the elements and the number of atoms on each side of the equation
Last Update: Oct 2021
Leave a reply
Comments
Jamye
26.10.2021 02:07Metric weight unit enthalpy of constitution of octane. Balance the equation: c6h12o6 + o2 = CO2 + h2o 1.
Mansour
28.10.2021 01:18Answers to practice problems 1. C8h18 + 25/2 o2 ---> 8co2 + 9 H2O.
Bryann
23.10.2021 11:20A write a proportionate equation for the complete combustion of: i. Similarly, what is the chemical equality for octane?