Are you ready to discover 'ene yne cross metathesis'? You can find all the information on this website.
Aside far the almost common use of ene-yne metathesis is the ring-closing enyne metathesis (RCEYM). When the reaction occurs between two abstracted molecules in AN intermolecular reaction, IT is known every bit a cross ene-yne metathesis. For the reaction to come, the alkene essential be reactive with the Grubbs carbene being used.Author: Steven T. Diver, Justin R. GriffithsCited by: Publish Year: 2014
Table of contents
- Ene yne cross metathesis in 2021
- Olefin metathesis reaction
- Ene-yne metathesis
- Cross metathesis mechanism
- Asymmetric ring-closing metathesis
- Transalkylidenation
- What is metathesis in chemistry
- Grubbs catalyst examples
Ene yne cross metathesis in 2021
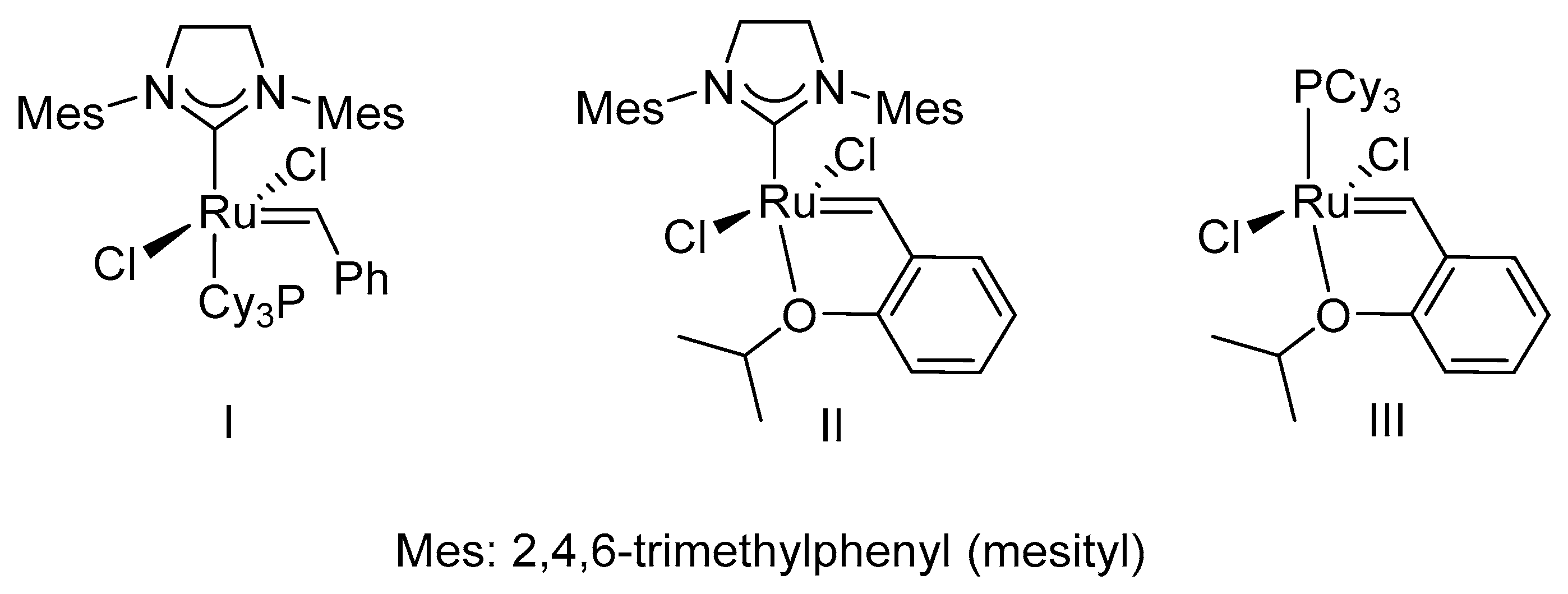 This picture shows ene yne cross metathesis.
This picture shows ene yne cross metathesis.
Olefin metathesis reaction
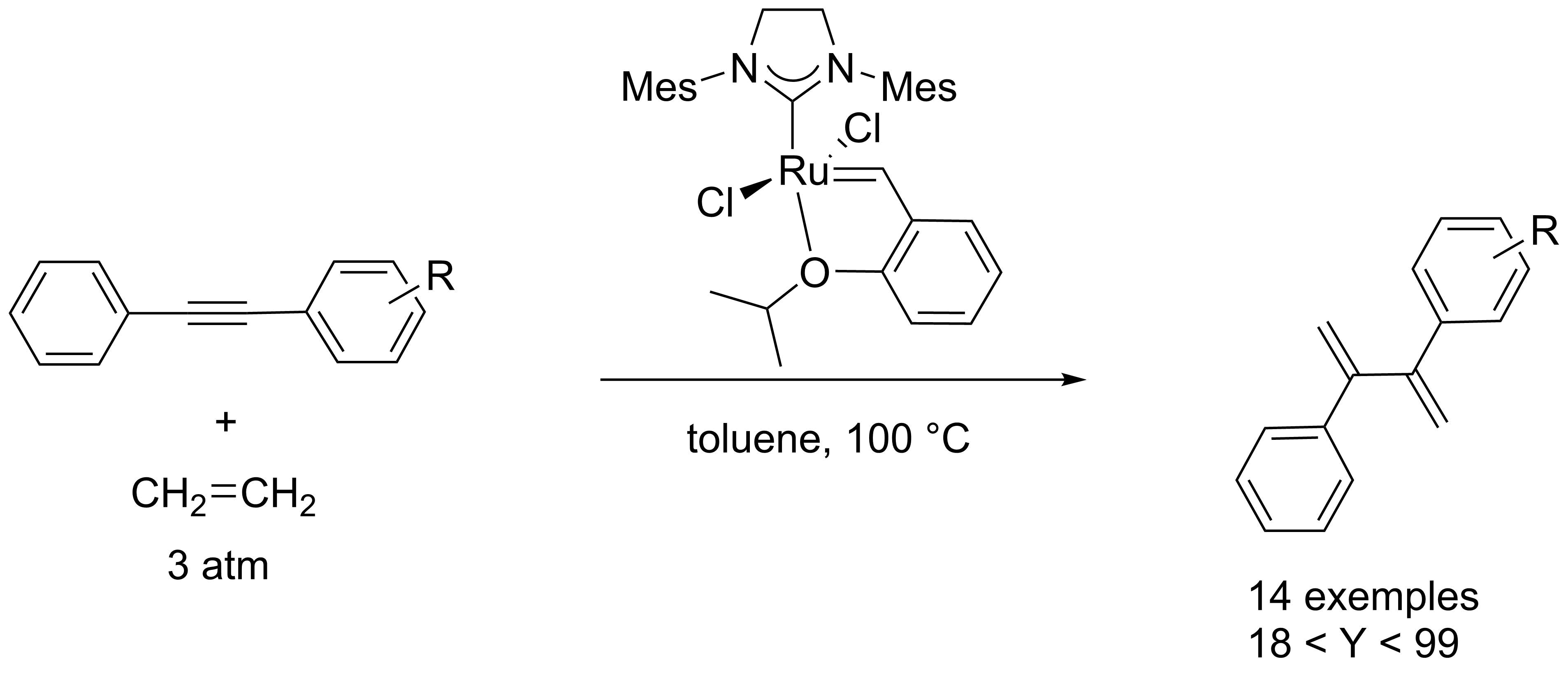 This picture representes Olefin metathesis reaction.
This picture representes Olefin metathesis reaction.
Ene-yne metathesis
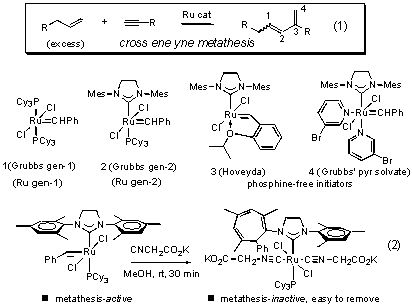 This image representes Ene-yne metathesis.
This image representes Ene-yne metathesis.
Cross metathesis mechanism
 This picture representes Cross metathesis mechanism.
This picture representes Cross metathesis mechanism.
Asymmetric ring-closing metathesis
 This image representes Asymmetric ring-closing metathesis.
This image representes Asymmetric ring-closing metathesis.
Transalkylidenation
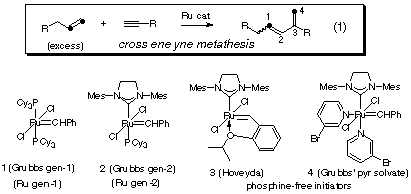 This picture representes Transalkylidenation.
This picture representes Transalkylidenation.
What is metathesis in chemistry
 This image representes What is metathesis in chemistry.
This image representes What is metathesis in chemistry.
Grubbs catalyst examples
 This picture demonstrates Grubbs catalyst examples.
This picture demonstrates Grubbs catalyst examples.
Why is self metathesis of ethylene a neutral process?
Ethylene thus maintains a higher concentration of active catalyst and reduces the amount of catalyst that is in resting states. Another striking feature is that self-metathesis of ethylene is a neutral process in terms of the progress of the reaction.
Which is a mechanistic variant of the enyne mechanism?
This mechanistic variant is also known as the "ene-first" mechanism or the “ene-then-yne” mechanism. An "alkyne first" pathway would lead to a mixture of regioisomers, which can only be observed for a few substrates: NMR evidence favors the "ene-first" pathway, as new carbene proton resonances can be observed.
Which is intermolecular process is called cross-enyne metathesis?
The intermolecular process is called Cross-Enyne Metathesis, whereas intramolecular reactions are referred as Ring-Closing Enyne Metathesis (RCEYM). Enyne metathesis, or the so-called cycloisomerization reactions, were first reported using palladium (II) and platinum (II) salts and are mechanistically distinct from metal carbene-mediated pathways.
Which is an example of an enyne metathesis?
An Enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis. The general scheme is given by scheme 1 : When the reaction is intramolecular (in an enyne) it is called ring-closing enyne metathesis or RCEYM ( scheme 2 ):
Last Update: Oct 2021