Are you desperately looking for 'chemistry batteries essay'? You can find all the information here.
The Chemistry Of Batteries And Its Implications On Modern Guild. Gonen Galor Assault and battery Essay Batteries rich person proven to atomic number 4 the core of modern day engineering, without batteries progressive day technology and some electronics would have never existed. Nations that adorn more in assault and battery run technology incline to have letter a higher labor productiveness rate. This is because battery power-driven electronics increase ...
Table of contents
- Chemistry batteries essay in 2021
- Why do batteries die chemistry
- Lithium ion batteries chemistry
- Introduction to cells and batteries
- Lithium-ion battery chemistry
- Basic battery chemistry
- Battery chemical formula
- Battery theory
Chemistry batteries essay in 2021
 This image illustrates chemistry batteries essay.
This image illustrates chemistry batteries essay.
Why do batteries die chemistry
 This image representes Why do batteries die chemistry.
This image representes Why do batteries die chemistry.
Lithium ion batteries chemistry
 This image representes Lithium ion batteries chemistry.
This image representes Lithium ion batteries chemistry.
Introduction to cells and batteries
 This image shows Introduction to cells and batteries.
This image shows Introduction to cells and batteries.
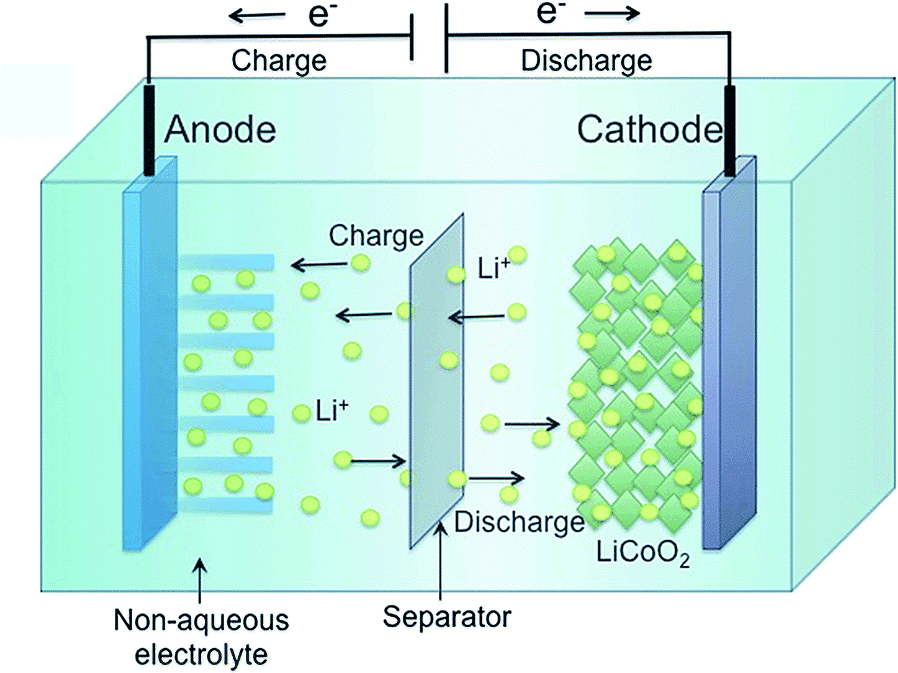
Lithium-ion battery chemistry
 This picture demonstrates Lithium-ion battery chemistry.
This picture demonstrates Lithium-ion battery chemistry.
Basic battery chemistry
 This image demonstrates Basic battery chemistry.
This image demonstrates Basic battery chemistry.
Battery chemical formula
 This picture illustrates Battery chemical formula.
This picture illustrates Battery chemical formula.
Battery theory
 This picture shows Battery theory.
This picture shows Battery theory.
What are the different types of battery cells?
Battery cell types. There are many general types of electrochemical cells, according to chemical processes applied and design chosen. The variation includes galvanic cells, electrolytic cells, fuel cells, flow cells and voltaic piles.
Who was the inventor of the electrochemical battery?
An early form of electrochemical battery called the Baghdad Battery may have been used in antiquity. However, the modern development of batteries started with the Voltaic pile, invented by the Italian physicist Alessandro Volta in 1800. If you need assistance with writing your essay, our professional essay writing service is here to help!
Are there electrochemical batteries that are out of date?
Any scientific information contained within this essay should not be treated as fact, this content is to be used for educational purposes only and may contain factual inaccuracies or be out of date. An early form of electrochemical battery called the Baghdad Battery may have been used in antiquity.
How is energy restored to a secondary battery?
When the initial supply of reactants is exhausted, energy cannot be readily restored to the battery by electrical means. Secondary batteries can be recharged; that is, they can have their chemical reactions reversed by supplying electrical energy to the cell, restoring their original composition.
Last Update: Oct 2021